
Laboratory-grown skin grafts are already part of the medical toolkit. However, a project from Tel Aviv University, supported by Zimin Institutes, promises a breakthrough in near-authentic skin transplant: dense, elastic, and multi-layered. In experimental mice, hair even grows at the burn site. T-Invariant explores how this technology was developed and how close it is to clinical application.
A white, thin sheet glimmers in the light. It looks and feels similar to the backing of children’s stickers. In this case, though, the sheet itself actually is the sticker, attached to a foil backing.
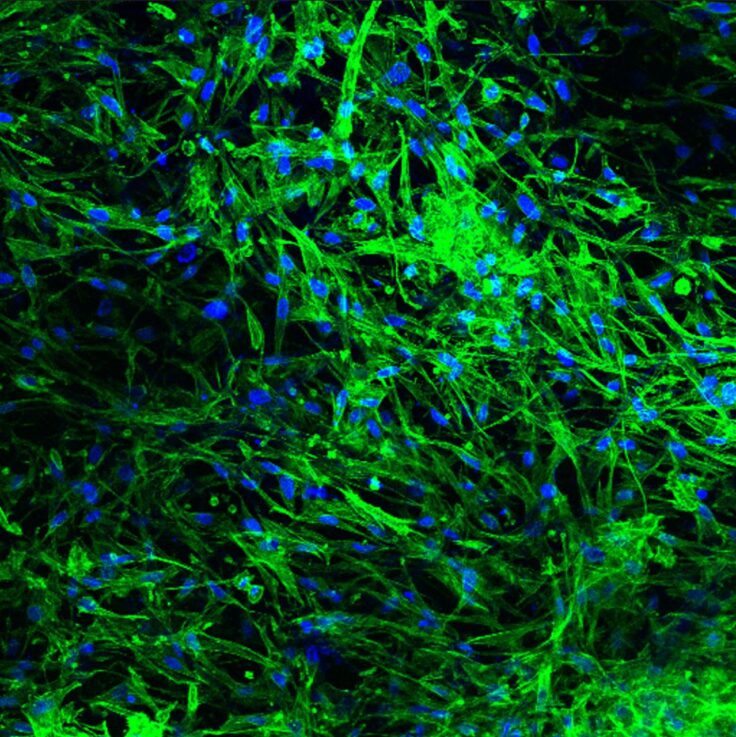
Dana Cohen-Gerassi lifts it with her fingernail, and the white sheet peels away, leaving delicate, web-like threads stretching toward the foil. In the case of a sticker, this would be glue, but here we see the nano-fiber itself — the scaffold for the future artificial skin, composed of multiple layers of ultra-fine threads.
In the next room, incubators maintain precise conditions (37.1°C and 5% carbon dioxide) where mouse cells are cultured in Petri dishes. These cells are nourished with a specialized solution of micro- and macronutrients supplemented with antibiotics. Each dish cultivates two cell types simultaneously: fibroblasts (the most common cells in connective tissue) and keratinocytes, the foundation of the epidermis, the skin’s outermost layer.
The cultured cells are then transferred using a pipette and seeded onto the nanofibrous sheet at a density of about 2,000 cells per square centimeter. The sheet, now seeded with cells, is returned to the incubator. After some time, it becomes a transplant ready for grafting. It looks nearly identical to the sheet I was just shown.
“It’s just not as white and it’s slightly more transparent,” Dana explains. “The living cells aren’t visible to the naked eye.”
Yet, after transplantation, the sheet gradually transforms into nearly normal skin. Various cell types migrate into it, developing into hair follicles and sweat glands.
“Look at a typical burn scar,” Dana says. “No hair grows there, and there are no sweat glands. It’s scar tissue. Now, look at the photos of our mice.”
Her claims are supported by a study published this summer in Advanced Functional Materials. “Histological analysis revealed early-stage formation of hair follicles and glands,” the paper states, accompanied by photographs.
“Our work is critical for patients with burns covering 50% or more of their body surface area,” says Professor Lihi Adler-Abramovich, one of the study’s lead co-authors.
In less severe cases, autotransplantation—grafting a patient’s own skin—is possible. But for extensive burns, cultured autografts are currently used, which involve growing fragments of the epidermis from the patient’s cells. This technology already saves countless lives.
“But it has many drawbacks. We learned of these challenges from Ayelet Di Segni at the Chaim Sheba Medical Center (Tel HaShomer), who grows transplants for patients,” Professor Adler-Abramovich explains. “We decided we could make the process simpler and more effective. That’s how this project started.”
Skin with Challenges
On April 12, 2021, the eve of Israel’s Memorial Day for fallen soldiers, Itzik Saidian, a veteran of the Golani Brigade, set himself on fire in front of the IDF’s Rehabilitation Department in Petah Tikva. He was protesting the insufficient support provided to soldiers like him, who suffer from post-traumatic stress disorder (PTSD) from their service.
Top news on scientists’ work and experiences during the war, along with videos and infographics — subscribe to the T-invariant Telegram channel to stay updated.
His act sparked widespread protests, leading to reforms and increased funding for the rehabilitation of military psychological trauma. Remarkably, Saidian, who suffered burns over 98% of his body, was saved.
He became the sixth and most high-profile patient at the Chaim Sheba Medical Center (Tel HaShomer) to receive lab-grown epidermal grafts. As Ayelet Di Segni told the newspaper Haaretz, around 600 grafts, covering a total of two square meters, were created for Saidian. The cells for these grafts were sourced from the only unburned parts of his body — his feet.
Ayelet, another co-author of the study, holds two roles at the medical center. She manages the tissue donor bank and oversees the laboratory that grows epidermal grafts. These roles are interconnected because, even with modern techniques, cadaveric skin grafts remain essential.
When a patient with extensive burns arrives, they first receive cadaver skin grafts, Ayelet explains. These grafts last about two weeks before rejection begins. If the burn is not too severe, doctors can then transplant the patient’s own skin. For larger burns, they try to buy time until lab-grown epidermal grafts are ready.
Producing these grafts typically takes three weeks. From one square centimeter of donor cells, Ayelet’s lab can grow about 50 square centimeters of epidermis in a Petri dish. However, once removed, the graft can shrink dramatically — to as little as one-fifth its original size. The epidermis is only the outermost layer of skin, and it lacks the elasticity and structural support provided by deeper layers.
“This is one of the main issues with the technology. Another is that these thin grafts are extremely difficult to handle. A third is the long time it takes to grow the cells,” Professor Adler-Abramovich explains. “Israel is a small country, which fosters collaboration. We met Ayelet, she shared these challenges, and we realized the graft needed a robust, supporting scaffold. We realized a scaffold technology, initially developed for bone regeneration, could be adapted for skin.”
Bio-Inspired Materials
Lihi, a biotechnologist by training, earned her PhD studying nanopeptides. Since 2015, she has led a group at Tel Aviv University developing bio-inspired materials. Her team includes materials scientists, engineers, biologists, doctors, and chemists. Dana Cohen-Gerassi, her doctoral student, is trained as an engineer specializing in materials.
Two of the group’s earlier developments have demonstrated significant promise. First, a superhydrophobic surface inspired by the structure of a lotus leaf, covered with tiny nano-needles that prevent bacterial adhesion. This technology led to dental materials that substantially reduce the risk of secondary caries, as confirmed by research. The second breakthrough involves nano-scaffolds for bone regeneration, drawing on the bone’s natural repair process.
“Bones have a natural ability to heal,” Professor Adler-Abramovich explains. “We studied how bones recover from small fractures. The first stage involves forming a scaffold in the extracellular matrix. We scaled this process up for severe fractures. That project, in collaboration with the European Commission, has advanced to trials on large animals.”
For skin, they developed a similar scaffold (adjusted for different physical properties). Both scaffolds are based on short RGD peptides. For bones, polysaccharides were added to create a hydrogel, but for skin, they used electrospinning to produce a material composed of ultra-fine, strong, yet elastic fibers.
Up-to-date videos on science during wartime, interviews, podcasts, and streams with prominent scientists — subscribe to the T-invariant YouTube channel!
RGD is a short amino acid sequence (arginine-glycine-aspartic acid), a component of the natural extracellular matrix. This inherently improves cell adhesion to the scaffold.
Another key detail: aspartic acid, a component of the sweetener aspartame, is already mass-produced. This means skin scaffolds could also be manufactured at scale, notes Lihi Adler-Abramovich.
Cells are applied to the scaffold in a mixed state, and then they spontaneously self-organize.
“It’s fascinating to watch,” the professor continues. “Keratinocytes gather on the upper layer, while fibroblasts settle on the lower layer, just like in healthy skin.”
The exact mechanism behind this is still under study. Lihi suggests one possible explanation: fibroblasts proliferate faster, while keratinocytes grow more slowly.
The researchers’ approach paid off. First, the new graft doesn’t shrink, allowing transplantation directly from the Petri dish to the test animal. This is critical, as any loss of surface area means lost time, which is vital for such patients.
The team also gained a slight edge in speed, though the details remain unclear. Since the scaffold has a predefined size, the key question is whether it has enough cells for transplantation.
“We’re currently growing a 10-by-10-centimeter graft in two weeks. But we suspect 4-5 days might be enough,” Dana Cohen-Gerassi speculates.
Most importantly, the regeneration process is significantly accelerated. In mouse experiments, healing occurred nearly twice as fast as with epidermal grafts.
“It’s remarkable,” says Professor Adler-Abramovich. “The tissue quality is also better. After healing, the burn site resembles healthy mouse skin, not scar tissue. It’s elastic and looks very good. Of course, I can’t guarantee the same results in humans.”
What’s Next?
So far, the new grafts have only been tested on mice. The next step is pigs, whose skin closely resembles human skin. In Israel, sourcing pigs for research is both challenging and costly. The lab is currently seeking academic funding for this phase.
Human trials are likely 5–10 years away, Lihi Adler-Abramovich estimates. While awaiting funding and further experiments, the lab is pursuing several refinements to the technology. First, it could potentially treat other skin injuries, such as diabetic ulcers. Second, the scaffold is not yet biodegradable; it becomes part of the tissue after healing. Another research avenue is programmable degradation.
A third experiment, underway now, could lead to a revolution in burn treatment. The team aims to embed biodegradable sensors directly into the skin graft scaffold.
“One of the most painful aspects of burn treatment is monitoring the healing process,” Dana Cohen-Gerassi explains. “Doctors periodically remove bandages to inspect the wound. This is not only excruciating but also hampers healing. To eliminate these checks, we’ve developed special prototype sensors that can function within the graft.”
The sensors are in the next room, but the team is preparing to file a patent, so they’re not shown to me.